Chemical bonds: definition, types, and examples How to predict number of bonds each element forms – chemsimplified Chlorine combined with two negative atom or 1 positive and other
Chapter 5.6: Properties of Polar Covalent Bonds - Chemistry LibreTexts
Covalent types properties bonding electrons atoms stable
How many valence electrons does cl have
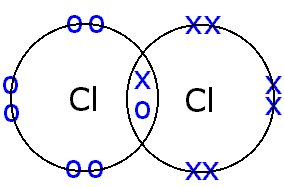
Valence electrons numerade elementChlorine cl2 atom molecule covalent atoms socratic electrons Covalent bondingBonding covalent lewis structures bond electrons chlorine libretexts sharing introduction chemical pair electron atom shared each chemistry chem molecular cl.
Bonds many chemical bonding chapter atom ppt powerpoint presentation number valence needsPeriodic table compounds chemistry ionic bonds covalent valence each family ions element elements electron lewis molecular has symbols dot columns The weakest c-cl bond is present inBonds honc predict caution rows works.

Covalent bond- definition, properties, types, examples
Pi bonds overlap draw side knowBiochemistry glossary: bonds 3.3: covalent bondingCovalent bonds ionic bonding libretexts chapter polarity atoms electrons electron molecular purely structures.
Igcse chemistry notes : chapter 3: bondingBonding covalent structure chlorine molecule atoms two chloride electrons hydrogen bond cl2 chemistry non sharing pair formed c3 gif metals Chapter 5.6: properties of polar covalent bondsBond weakest present.